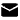ADC (Antibody-Drug Conjugates) are a class of targeted cancer therapies that combine the specificity of monoclonal antibodies (mAbs) with the potent cytotoxic effects of small-molecule drugs. The goal of ADCs is to deliver chemotherapy directly to cancer cells, minimizing the effects on healthy cells and improving the therapeutic index (the ratio of effective dose to toxic dose). They are designed to selectively target cancer cells while sparing normal tissues, making them more effective and less toxic than conventional chemotherapy.
ADC Development Process
Developing ADCs involves several key steps:
Antigen Selection: One of the biggest challenges in ADC development is selecting a suitable target antigen. The target antigen should be highly expressed on cancer cells but minimally expressed on normal cells to reduce off-target toxicity. The antigen should also be internalized after binding to the antibody to allow for effective drug delivery.
Antibody Selection and Engineering: The monoclonal antibody used in the ADC must have high affinity and specificity for the chosen antigen. Additionally, the antibody's pharmacokinetics, immunogenicity, and ability to activate the immune system are important considerations.
Linker Development: The linker is crucial for the efficacy and safety of the ADC. A stable linker ensures that the cytotoxic drug does not get released prematurely in the bloodstream, while a cleavable linker ensures the drug is released in the target cell, where it will be most effective. Linkers must be designed to balance stability, drug release, and ease of conjugation to the antibody.
Cytotoxic Drug Selection: The cytotoxic drug must be potent enough to kill cancer cells at low doses but ideally not toxic to healthy cells. Common cytotoxic agents used in ADCs include:
·Maytansinoids (e.g., DM1) – microtubule inhibitors
·Auristatins (e.g., MMAE) – disrupt microtubule polymerization
·Calicheamicins – DNA-damaging agents
·Duocarmycins – DNA-alkylating agents
Conjugation: Once the antibody, drug, and linker are selected, the cytotoxic drug is conjugated to the antibody. This is often done using chemical linkers that can covalently attach the drug to the antibody via the linker. The number of drugs attached per antibody is also important, as too many drugs can reduce the ADC’s stability or increase its toxicity, while too few drugs may reduce its effectiveness.
Preclinical and Clinical Testing: ADCs undergo extensive preclinical testing in animal models to assess their efficacy, safety, pharmacokinetics, and pharmacodynamics. Once proven effective, they enter clinical trials. This phase involves testing the ADC in human subjects for safety, dose escalation, optimal dosing regimen, and clinical efficacy.
Examples of Approved ADCs
Several ADCs have been approved for use in cancer treatment, and more are in development:
1.Trastuzumab emtansine (Kadcyla): An ADC combining trastuzumab (an anti-HER2 antibody) with DM1, a potent antimicrotubule agent. It is used to treat HER2-positive breast cancer.
2.Brentuximab vedotin (Adcetris): This ADC targets CD30, a cell surface protein expressed on certain lymphoma cells, and delivers MMAE (a microtubule-disrupting drug). It is used to treat Hodgkin lymphoma and systemic anaplastic large cell lymphoma.
3.Inotuzumab ozogamicin (Besponsa): An ADC targeting CD22 on B-cell malignancies, delivering calicheamicin, a potent DNA-damaging agent. It is approved for use in acute lymphoblastic leukemia (ALL).
4.Polatuzumab vedotin (Polivy): An ADC targeting CD79b, used in combination with other therapies for certain types of B-cell lymphoma.
Challenges in ADC Development
1.Off-Target Toxicity: Despite the specificity of the antibody, off-target effects can still occur, leading to side effects. The drug must be selectively delivered to cancer cells without harming normal tissue.
2.Antigen Heterogeneity: Tumors often have heterogeneous of the target antigen, which can lead to incomplete targeting of all cancer cells. Some tumor cells may not express enough of the target antigen for effective therapy.
3.Resistance Mechanisms: Over time, tumor cells may develop resistance to ADCs by altering the target antigen, reducing the internalization of the ADC, or activating cellular repair mechanisms to counteract the cytotoxic effects.
4.Manufacturing Complexity: The production of ADCs involves complex processes, and maintaining consistency and quality control in manufacturing can be challenging.
Future Directions
·Expanded Antigen Targets: Researchers are working on identifying new cancer-specific antigens and optimizing ADCs for rare or difficult-to-treat cancers.
·Enhanced Linkers and Drugs: New linker technologies and more potent cytotoxic agents are being explored to improve the effectiveness and safety profiles of ADCs.
·Combination Therapies: Combining ADCs with other treatment modalities, such as immune checkpoint inhibitors, chemotherapy, or targeted therapy, is being studied to enhance their efficacy and overcome resistance mechanisms.
ADCs represent an exciting and rapidly growing field in cancer therapy, offering a promising way to target tumors more precisely and minimize the side effects of conventional chemotherapy.

 +8613708084407
+8613708084407