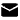Weight management has been incorporated into the national strategy, and the GLP-1 market continues to be extremely popular.
To implement the Healthy China Action (2019 - 2030), promote a healthy lifestyle, and prevent and control overweight and obesity, the national government has recently introduced a series of important policies to elevate weight management to an unprecedented strategic level:
- On June 6, 2024, the National Health Commission released a notification on the implementation plan for the "Weight Management Year" activity.
- On December 31, 2024, the General Office of the National Health Commission released the Weight Management Guidelines (2024 Edition).
- On April 11, 2025, the Medical Administration Department issued a notice on the establishment and management of healthy weight management clinics.
- On April 14, 2025, the Planning, Development, and Informatization Department issued a notice on incorporating the Healthy Weight Management Action and two other actions into the Healthy China Action.
With weight management now part of the national strategy, this field is receiving unprecedented attention. GLP-1 drugs achieved over 50 billion US dollars in sales in 2024, with transaction volumes continuously reaching new highs. According to PharmaMagic, the global transaction volume of GLP-1 drugs in the first quarter of 2025 has approached the total volume of 2024, reflecting the sustained market interest.

Figure 1: An overview of GLP-1 class of antidiabetic and weight - loss drug transactions. Source: PharmaMagic
In this global competition among pharmaceutical giants, Eli Lilly's dual - target drug Tirzepatide has shown great brilliance. Recently, the world's richest man, Musk, revealed on social media that his weight - loss plan has switched from Semaglutide to Tirzepatide, calling it a gift from science to humanity. Why can Tirzepatide stand out in the billion - dollar GLP - 1 track? Science and data reveal the answer.
The innovative dual - target design mechanism achieves a "dimensional attack."
Tirzepatide, the world's first approved dual - receptor agonist for both Glucagon - like Peptide - 1 (GLP - 1) and Glucose - dependent Insulinotropic Polypeptide (GIP), represents a major breakthrough in the treatment of diabetes and obesity. Composed of 39 amino acids with the molecular formula C225H348N48O68, its dual - target innovative design lays the foundation for its excellent clinical efficacy:
- Retain GIP high - activity amino acids: Tyr10, Ile12, Asp15, Lys16, IIe17, Gln19, Val23, Ala28
- Retain Gly4 (Gly10), Phe6 (Phe12), Thr7 (Thr13), Asp9 (Asp15) and Phe22 (Phe28) to ensure GLP - 1 receptor activity.
- The C - terminal's 11 amino acids are derived from Exenatide, enhancing the peptide's helicity and structural stability through molecular interactions.
- Replace the natural amino acid at position 2 with Aib (2 - aminoisobutyric acid) to avoid inactivation by DPP - 4 (dipeptidyl peptidase - 4) in the body.
- C20 fatty diacids are connected to the peptide chain's Lys at position 20 via one Glu and two AEEA linkers, extending the in - body half - life to nearly 5 days.
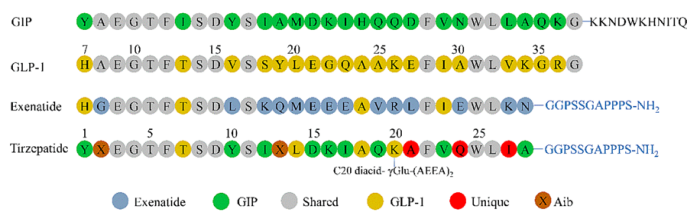
Figure 2: The structures of GLP - 1 (7 - 37), GIP (1 - 42), Exenatide, and Tirzepatide. Source: DOI:10.1016/j.bmc.2024.117630
As an innovative single - molecule dual - receptor agonist, Tirzepatide breaks the limitations of traditional single - target drugs. By simultaneously activating GIP and GLP - 1 receptors, it produces a synergistic effect of "1 + 1 > 2," showing unique systemic bolic regulation. Its blood - sugar - lowering mechanism mainly involves glucose - dependent blood - sugar reduction, avoiding hypoglycemia risks. It also inhibits glucagon secretion, reducing liver glycogenolysis and gluconeogenesis. As a dual GIP and GLP - 1 agonist, it promotes the proliferation and survival of pancreatic β - cells, improves insulin secretion, and helps lower blood sugar. Weight loss is achieved through four main ways:
- Suppressing appetite and increasing satiety: Both GIP and GLP - 1 receptors act on hypothalamic and other appetite - regulating centers, reducing hunger and appetite while enhancing satiety and lowering food intake.
- Delaying gastric emptying: Activating receptors slows gastric emptying, prolonging food retention in the stomach, enhancing satiety, and reducing eating frequency.
- Regulating food preferences and reward pathways: Activating GLP - 1 receptors alters the brain's food - related reward responses, reducing preference for high - calorie foods and cutting energy intake.
- Improving energy bolism: Regulating fat bolism and energy consumption, promoting fat breakdown, and reducing fat accumulation.
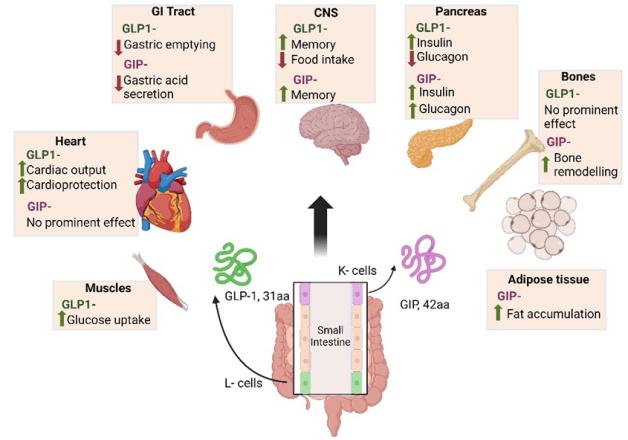
Figure 3: The mechanism of action of Tirzepatide. Source: ACS American Chemical Society's official account
Head - to - head clinical trials show overwhelming clinical advantages.
Since starting Tirzepatide's first clinical trial on May 3, 2016, Lilly has conducted over 200 clinical studies worldwide, showing strong R & D capabilities. Among these, several head - to - head trials against semaglutide are particularly significant, offering strong evidence for Tirzepatide's clinical benefits.
The pivotal Phase III clinical trial (NCT03987919), a multicenter, randomized, and controlled study, assigned patients in a 1:1:1:1 ratio to receive weekly Tirzepatide (5mg, 10mg, or 15mg) or Semaglutide (1mg) on a metformin therapy basis. The primary endpoint was to evaluate whether the A1c (glycated hemoglobin) improvement of Tirzepatide 10mg and 15mg groups at week 40 was non - inferior to Semaglutide. Key secondary endpoints included:
- The non - inferiority of Tirzepatide 5mg in A1C reduction.
- The superiority of the three Tirzepatide doses in A1C and weight loss, and a higher proportion of subjects achieving an A1C <7% target.
- A higher proportion of subjects achieving an A1C <5.7% in the Tirzepatide 10mg and 15mg groups compared to Semaglutide.
Figure 4: Results of the NCT03987919 clinical trial. Source: Eli Lilly and Company
Another Phase III clinical trial (NCT05822830) assessed the efficacy and safety of Tirzepatide versus Semaglutide in obese or overweight adults without diabetes but with at least one comorbidity (e.g., hypertension, dyslipidemia, obstructive sleep apnea, cardiovascular disease). Patients received Tirzepatide (max tolerated dose of 10mg or 15mg) or Semaglutide (1.7mg or 2.4mg). At week 72, all primary and five key secondary endpoints favored Tirzepatide. It achieved an average weight loss of 20.2%, surpassing Semaglutide's 13.7%, with a 1.47 - fold higher weight - loss multiple. In the Tirzepatide group, 31.6% of patients lost at least 25% of their weight, versus 16.1% in the Semaglutide group. Both groups reported mild - to - moderate gastrointestinal adverse events as the most common safety issues.

Figure 5: Results of the NCT05822830 clinical trial. Source: Eli Lilly and Company
These landmark studies not only solidify Tirzepatide's leading position in bolic disease treatment but also set a new benchmark for obesity treatment with its breakthrough weight - loss effects, offering patients a better therapeutic option.
Market Impact: A "duel" between corporate giants.
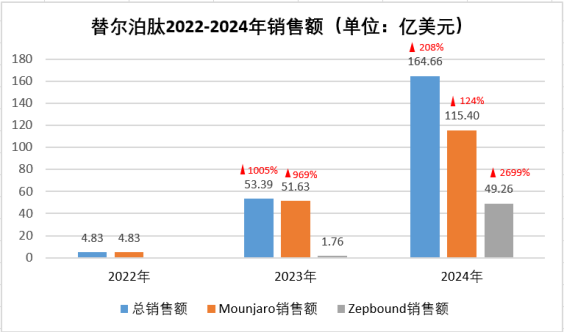
Figure 6: Tirzepatide's sales from 2022 to 2024
Tirzepatide's commercialization has set new records in the global bolic treatment field. In May 2022, its antidiabetic version, Mounjaro, was approved by the FDA for type 2 diabetes treatment. It showed remarkable market power, with sales soaring to 5.163 billion US dollars in 2023, a year - on - year increase of 969%, far outpacing Semaglutide's early - stage performance (286 million US dollars in 2018, 1.686 billion US dollars in 2019, +490%). By the end of 2023, Tirzepatide's weight - loss version, Zepbound, was approved by the FDA. It quickly gained traction, achieving 176 million US dollars in sales in just two months. In 2024, Tirzepatide fully erupted, with annual sales reaching 16.466 billion US dollars, a year - on - year growth of 208%, becoming the second antidiabetic and weight - loss drug after Semaglutide to exceed 10 billion US dollars in annual sales. The antidiabetic Mounjaro achieved 11.54 billion US dollars in sales, a year - on - year growth of 124%, continuing to lead the diabetes treatment market. The weight - loss Zepbound reached 492.6 million US dollars, showing huge market potential. These impressive commercial performances not only confirm the clinical advantages of Tirzepatide's dual - target mechanism but also reshape the competitive landscape of the global bolic disease treatment market.

Figure 7: Tirzepatide's approval for the OSA indication. Source: ACS American Chemical Society's official account
According to PharmaMagic, Tirzepatide's clinical research now covers 23 indications, showing strong potential for indication expansion. Three indications have been approved, and in February 2025, an application for heart failure with preserved ejection fraction was submitted. Additionally, six indications, including cardiovascular risk, psoriatic arthritis, and bronchial asthma, are in smooth Phase III clinical progress. In the future, they may broaden Tirzepatide's clinical applications, offering more breakthrough treatment options for global patients.
Currently, Novo Nordisk and Eli Lilly dominate nearly 95% of the GLP - 1 drug market. Despite Semaglutide's leading 2024 sales, its production capacity constraints and patent expiration in 2026 may hinder its future development. In contrast, Eli Lilly's Tirzepatide, with its excellent clinical efficacy, unique dual - target mechanism, and differentiated indication layout, shows stronger growth potential and is expected to continue leading in bolic treatment.
Pu-Kang Biotech: A high - technology - barrier peptide drug R & D platform.
As the world's first GLP - 1 / GIP dual - target receptor agonist, Tirzepatide, with its breakthrough mechanism and excellent clinical efficacy, has become a revolutionary drug in bolic disease treatment. Despite competition from Semaglutide and new antidiabetic and weight - loss drugs, Tirzepatide's unique dual - target mechanism and expanding indications will maintain its market leadership. Looking ahead, with the penetration of multi - target drug R & D technology and the development of emerging markets, the GLP - 1 drug market will embrace broader development prospects.
Founded in 2008, Chengdu Pu-Kang Biotechnology Co., Ltd. (https://peglinke.com/about.html#pk_1) has been dedicated to the peptide field. It provides global pharmaceutical and biotech companies with peptide drug fragment synthesis and long - acting modification solutions. With world - leading peptide production and development processes, Pu-Kang Biotech focuses on high - technology - barrier peptide API R & D and production. Its experienced and innovative R & D team meets various customer needs, from custom synthesis to bulk production. Since 2017, it has supported several overseas pharmaceutical companies by supplying Semaglutide and Tirzepatide side chains and related short peptides. d on QbD, it has completed the pharmaceutical research of these side chains and short peptides and written complete DMF documents. Upholding people - oriented, integrity - d, quality - first, and innovation - driven principles, Pu-Kang Biotech strives to become China's leading peptide brand, offering higher - quality products and more professional services.

 +8613708084407
+8613708084407