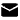CAS:1446013-08-6
Are you ready to unlock the full potential of Fmoc-His-Aib-OH TFA? Look no further because this ultimate guide will unveil its potent benefits and revolutionary applications. Whether you're a seasoned researcher or a curious mind, this article will provide you with the knowledge you need to harness the power of this compound.
Fmoc-His-Aib-OH TFA, a key ingredient in various industries, offers a wide array of advantages. From its exceptional stability to its enhanced solubility in common organic solvents, this compound boasts unique characteristics that set it apart from the rest. By understanding its properties and applications, you can explore breakthrough possibilities in drug delivery systems, peptide synthesis, and much more.
In this comprehensive guide, we will delve into the impressive benefits of Fmoc-His-Aib-OH TFA and explore its role in various fields. We'll also shed light on the latest research and advancements, showcasing how this compound is pushing boundaries and revolutionizing industries.
So, if you're ready to embark on a journey of discovery and innovation, join us in unraveling the potential of Fmoc-His-Aib-OH TFA. The possibilities are limitless, and this guide will equip you with the knowledge to make the most of them.
Understanding the structure and properties of Fmoc-His-Aib-OH TFA
Fmoc-His-Aib-OH TFA, also known as 9-Fluorenylmethyloxycarbonyl-L-histidine-2-aminoisobutyric acid trifluoroacetate, is a versatile compound that has garnered significant attention in the scientific community. Its unique structure and properties make it a valuable tool in various applications, particularly in the fields of drug development and peptide synthesis.
At the core of Fmoc-His-Aib-OH TFA is the Fmoc (9-Fluorenylmethyloxycarbonyl) group, which serves as a protective group for the amino terminus of amino acids during peptide synthesis. The Fmoc group is known for its stability, ease of removal, and compatibility with a wide range of reaction conditions, making it a popular choice in solid-phase peptide synthesis (SPPS).
The incorporation of the L-histidine (His) and 2-aminoisobutyric acid (Aib) moieties within the Fmoc-His-Aib-OH TFA structure further enhances its properties. L-histidine, an essential amino acid, is known for its ability to participate in various biochemical processes, including pH regulation and l ion binding. The Aib residue, on the other hand, is a non-proteinogenic amino acid that is known to impart structural rigidity and stability to peptides, making it a valuable component in the design of peptide-d drugs and therapeutics.
The trifluoroacetate (TFA) counterion in Fmoc-His-Aib-OH TFA plays a crucial role in the compound's solubility and stability. TFA is a common counterion used in the synthesis and purification of peptides and other biomolecules, as it enhances the solubility of these compounds in common organic solvents, such as dichloromethane and dimethylformamide (DMF).
Overall, the unique structural features and properties of Fmoc-His-Aib-OH TFA make it a highly versatile and valuable compound in various scientific and industrial applications. Its ability to impart stability, solubility, and structural rigidity to peptides and other biomolecules has contributed to its widespread use in the development of innovative drug candidates and advanced peptide-d materials.
The role of Fmoc-His-Aib-OH TFA in drug development
The pharmaceutical industry has long recognized the potential of Fmoc-His-Aib-OH TFA in the development of novel drug candidates. The compound's ability to enhance the stability and solubility of peptide-d drugs has made it a valuable tool in the quest for more effective and targeted therapies.
One of the primary applications of Fmoc-His-Aib-OH TFA in drug development is its use in the design and synthesis of peptide-d drugs. Peptides, which are short chains of amino acids, have emerged as promising therapeutic agents due to their high specificity, low toxicity, and potential for targeted delivery. However, the inherent instability and poor solubility of many peptides have been a significant challenge in their development as viable drug candidates.
By incorporating Fmoc-His-Aib-OH TFA into the peptide structure, researchers can overcome these limitations. The Fmoc group provides protection to the amino terminus, while the Aib residue enhances the structural rigidity and stability of the peptide. Additionally, the TFA counterion improves the solubility of the peptide in common organic solvents, facilitating the purification and formulation processes.
Furthermore, the unique properties of Fmoc-His-Aib-OH TFA have enabled the development of innovative drug delivery systems. Researchers have explored the use of this compound in the fabrication of nanoparticles, micelles, and other drug delivery vehicles that can selectively target diseased cells or tissues, thereby improving the therapeutic efficacy and reducing the side effects of the drug.
Applications of Fmoc-His-Aib-OH TFA in peptide synthesis
Peptide synthesis is a critical field in the life sciences, with applications ranging from drug development to the production of advanced materials. Fmoc-His-Aib-OH TFA has become a valuable tool in this domain, enabling researchers and manufacturers to overcome various challenges and unlock new possibilities.
One of the primary applications of Fmoc-His-Aib-OH TFA in peptide synthesis is its use as a building block in solid-phase peptide synthesis (SPPS). SPPS is a widely adopted technique for the assembly of peptides, where amino acids are sequentially added to a solid support, such as a resin. The Fmoc group in Fmoc-His-Aib-OH TFA serves as a protective group, ensuring the selective addition of amino acids and preventing unwanted side reactions during the synthesis process.
The incorporation of the Aib residue in Fmoc-His-Aib-OH TFA also plays a crucial role in peptide synthesis. Aib is known to promote the formation of secondary structures, such as α-helices and β-turns, within the peptide sequence. This structural rigidity can be beneficial in the design of peptide-d drugs, as it can enhance the stability and bioavailability of the peptide, ultimately improving its therapeutic efficacy.
Beyond its use in SPPS, Fmoc-His-Aib-OH TFA has also found applications in the synthesis of cyclic peptides. Cyclic peptides are a class of peptides with a closed loop structure, which can confer enhanced stability, improved target binding, and increased resistance to proteolytic degradation. The Fmoc group and the Aib residue in Fmoc-His-Aib-OH TFA facilitate the cyclization process, enabling the production of these specialized peptides with greater efficiency and precision.
In the field of peptide engineering, Fmoc-His-Aib-OH TFA has played a pivotal role in the development of innovative peptide-d materials. Researchers have explored the use of this compound in the synthesis of self-assembling peptides, which can be designed to form nanostructures with diverse applications, such as drug delivery, tissue engineering, and biosensing.
Furthermore, Fmoc-His-Aib-OH TFA has found applications in the production of peptide-d hydrogels, which are three-dimensional, water-swollen networks of peptides. These hydrogels have garnered significant interest in the biomedical field due to their potential for use in wound healing, tissue regeneration, and the controlled release of therapeutic agents.
The versatility of Fmoc-His-Aib-OH TFA in peptide synthesis has also extended to the field of peptide-d catalysts. Researchers have utilized this compound to synthesize peptide-d enzymes and biocatalysts, which can be employed in a wide range of industrial processes, from the production of fine chemicals to the development of sustainable biofuels.
In summary, Fmoc-His-Aib-OH TFA has become an indispensable tool in the realm of peptide synthesis, enabling researchers and manufacturers to overcome challenges, explore new frontiers, and develop innovative peptide-d products and technologies that have the potential to transform various industries and improve human well-being.

 +8613708084407
+8613708084407