
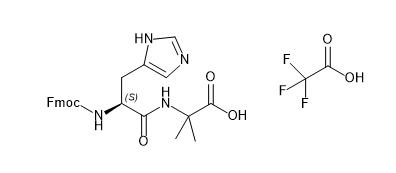
CAS:1446013-08-6
DMF Num:1446013-08-6
Fmoc Protection:Optimized for solid-phase peptide synthesis (SPPS), enabling efficient coupling in GLP-1 analog development.
Aib Residue:Introduces backbone rigidity, enhancing bolic stability in long-acting peptides.
Low Impurity Profile:Single impurity ≤0.5%, total impurities ≤1.0% (COA available upon request).
Semaglutide & GLP-1 Agonist Synthesis:Critical for constructing the His-Aib motif in Semaglutide's amino acid sequence, improving synthetic efficiency by 20%+ compared to standard derivatives.
Peptide Drug Discovery:Suitable for library screening in obesity, diabetes, and bolic disease research.
cGMP-Compliant Production:Scalable from 100 mg to 5 kg batches, supporting preclinical research to commercial manufacturing.
ISO 9001:2015 & ISO 14001 Certified:Manufactured in a cGMP-compliant facility with strict quality control.
Global Regulatory Support:Provides DMF, CE, and REACH registration documents for EU/US markets.
Stability Testing:Validated for 24 months under -20°C dry storage.
Custom Modifications:Alternative protection groups (e.g., Boc, Cbz) or isotopic labeling upon request.
Rush Services:24-hour turnaround for urgent orders (subject to availability).
Technical Consultation:Free peptide synthesis optimization by our PhD-level chemists.
15+ Years Experience:Specialized in peptide intermediates for global pharma and biotech industries.
R&D Capability:30+ scientists in a 2,000㎡ state-of-the-art laboratory.
Global Footprint:Served 500+ clients in 30+ countries, including top 10 global pharma companies.
Email:export@pu-kang.com
Phone:+86-137-0808-4407
Address:Zone 1 and 2, 1-3F, Building 3, Chengdu Cross-Strait Science and Technology Industrial Development Park [Jindi Weixin Wenjiang Intelligent Park], Wenjiang District, Chengdu, China.