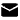Learn about Boc-Tyr(tBu)-Aib-OH (CAS 2639221-78-4), a Boc-protected dipeptide used in peptide synthesis. This article covers its structure (C22H34N2O6), IUPAC name, key functional groups, usage in research (e.g. Tirzepatide fragments), solubility and storage guidelines, and related peptide sequences.
Introduction
Boc-Tyr(tBu)-Aib-OH (CAS 2639221-78-4) is a protected dipeptide used widely in peptide synthesis. It combines N-Boc–protected tyrosine (with a tert-butyl group on the phenol) and the non-natural amino acid Aib (α-aminoisobutyric acid). In practice, chemists use it as a building block to introduce tyrosine and Aib residues while controlling reactivity. For example, this “Tirzepatide sidechain” is found in synthetic fragments of the diabetes drug tirzepatide. Chengdu Pukang Biotechnology Technology Co., Ltd. provides Boc-Tyr(tBu)-Aib-OH (Formula C22H34N2O6, MW 422.24) as a peptide intermediate. Its appearance is a white to off-white powder.
Chemical Structure and Properties
Boc-Tyr(tBu)-Aib-OH is a Boc-protected tyrosine–Aib dipeptide. Its full IUPAC name is 2-methyl-2-[[(2S)-2-[(2-methylpropan-2-yl)oxycarbonylamino]-3-[4-[(2-methylpropan-2-yl)oxy]phenyl]propanoyl]amino]propanoic acid. In simpler terms, the molecule has:
-
Molecular formula: C22H34N2O6
-
Molecular weight: about 422.5 g/mol
-
Key functional groups: a tert-butoxycarbonyl (Boc) carbamate protecting the amino terminus of Tyr, a tert-butyl (tBu) ether on the tyrosine phenol, a peptide amide bond between Tyr and Aib, and a free carboxylic acid at the C-terminus.
The Boc group on the tyrosine nitrogen and the tBu group on the phenolic oxygen make the dipeptide inert during chain assembly, to be removed later under acidic conditions. Aib (α-methylalanine) is a bulky, non-proteinogenic amino acid that promotes helical conformation in peptides. This structure is why researchers include Boc-Tyr(tBu)-Aib-OH when designing conformationally constrained or helical peptides.
Mechanism of Action and Applications
Boc-Tyr(tBu)-Aib-OH is not a drug but a synthetic intermediate in peptide chemistry. Its “mechanism” is simply to serve as a protected fragment during peptide assembly. In solid-phase peptide synthesis (SPPS), this dipeptide can be coupled as one piece. The Boc (tert-butoxycarbonyl) group protects the amino end of tyrosine, preventing unwanted reactions, while the tBu group shields the tyrosine’s hydroxyl group. After coupling, the Boc and tBu groups are removed (e.g. with trifluoroacetic acid) to free the amine and phenol for further chemistry.
Because Aib induces helices, peptides containing Boc-Tyr(tBu)-Aib-OH often form stable helices or turns. For example, Aib is deliberately introduced to improve peptide rigidity and bioactivity. This dipeptide appears in the synthesis of long-acting peptide drugs like tirzepatide (a diabetes medication). In tirzepatide fragments, the sequence Boc-Tyr(tBu)-Aib-… is used to incorporate tyrosine and helical constraints. More generally, researchers use it in designing novel peptides for drug delivery, oncology, or bolic disease research.
In short, Boc-Tyr(tBu)-Aib-OH’s “action” is to provide controlled reactivity and structural constraint in peptide chains. It is supplied by peptide synthesis companies such as Chengdu Pukang Biotechnology Technology Co., Ltd. as a reagent for research purposes.
Solubility and Storage
Proper solubility and storage are important for handling Boc-Tyr(tBu)-Aib-OH in the lab:
-
Solvents: It is very soluble in polar organic solvents like N,N‑dimethylformamide (DMF) and soluble in methanol. It is sparingly soluble in glacial acetic acid and only very slightly soluble in chloroform. It is essentially insoluble in water.
-
Storage: To maintain stability and purity, store Boc-Tyr(tBu)-Aib-OH at -20 °C in a freezer. Keep it dry and under inert atmosphere (e.g. nitrogen) if possible. Under these conditions, it remains stable for extended periods.
In practice, dissolve the powder in dry DMF or DMSO just before use in peptide coupling. After use, refrigerate and desiccate the residue. Always follow the specific guidelines from the supplier or safety datasheet.
Related Peptide Products and Sequences
Boc-Tyr(tBu)-Aib-OH is often incorporated into larger peptide fragments. One example includes:
-
Boc-Tyr(tBu)-Aib-Glu(OtBu)-Gly-OH (CAS 2682040-93-1): a protected tetrapeptide segment used in solid-phase synthesis. This compound has formula C33H52N4O10 and MW 664.37. This tetrapeptide includes the Boc-Tyr(tBu)-Aib motif as its N-terminal fragment.
These examples show how Boc-Tyr(tBu)-Aib-OH serves as a monomeric unit in the construction of larger, biologically active peptides. By using pre-made dipeptide segments, chemists can streamline synthesis and reduce side reactions.
References
-
PubChem: https://pubchem.ncbi.nlm.nih.gov/compound/2639221-78-4
-
ChemSpider: http://www.chemspider.com/Chemical-Structure.78203512.html
-
Chengdu Pukang Biotechnology Technology Co., Ltd.: https://peglinke.com/
-
SciFinder (CAS): https://scifinder.cas.org/ (subion required)

 +8613708084407
+8613708084407