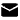Fmoc-Ile-Aib-OH (CAS 2171139-20-9) is an Fmoc‑protected L-isoleucine–Aib (α-aminoisobutyric acid) dipeptide used as a building block in peptide synthesis. It features a fluorenylmethoxycarbonyl (Fmoc) protecting group and an α-methylalanine residue, giving it unique structural properties. This article reviews its structure, function in SPPS, uses in peptide drugs (e.g. tirzepatide), solubility, storage, and related peptide sequences.
Introduction
Fmoc-Ile-Aib-OH is a specialized dipeptide intermediate commonly used in solid-phase peptide synthesis (SPPS). It combines L-isoleucine (Ile) and the non-standard amino acid α-aminoisobutyric acid (Aib) with an N-terminal 9‑fluorenylmethoxycarbonyl (Fmoc) protecting group. The Fmoc group shields the amino terminus during peptide coupling and is later removed by mild . This protected dipeptide is relevant in synthesizing peptides and peptide drugs, such as GLP-1 analogs and related therapeutics. Its CAS number is 2171139-20-9, and it is sold as a white to off-white powder. Product link from PegLinke
Structure and Chemical Information
FmoC-Ile-Aib-OH has the molecular formula C25H30N2O5 and a molecular weight of about 438.5 g/mol. Structurally, it consists of an Fmoc (fluorenylmethoxycarbonyl) group attached to the nitrogen of L-isoleucine, which is peptide-bonded to Aib (α-methylalanine) with a free carboxylic acid at the C-terminus (―OH). Its IUPAC/systematic name is “2-[(2S,3S)-2-({[(9H-fluoren-9-yl)methoxy]carbonyl}amino)-3-methylpentanamido]-2-methylpropanoic acid.” This name encodes the Fmoc urethane on isoleucine and the α-methylation of alanine. It is produced at high purity (≥98%) for research use.
Function as an Fmoc-Protected Dipeptide Building Block
Fmoc-Ile-Aib-OH serves as a protected dipeptide building block in peptide synthesis. The Fmoc group (9-fluorenylmethoxycarbonyl) is a common amine-protecting group in SPPS. It is stable to acids but is removed by mild (usually piperidine) without harming other acid-sensitive groups. This makes Fmoc chemistry ideal for stepwise peptide chain assembly on resin.
The Aib residue (α-aminoisobutyric acid), also known as α-methylalanine, is an α,α-disubstituted (non-proteinogenic) amino acid. Aib has two methyl groups on the α-carbon, which restricts backbone flexibility. Incorporating Aib in peptides strongly favors helical conformations. This feature is useful in designing peptidomimetics and drug candidates with enhanced stability and bioactivity.
Current Research and Uses
Fmoc-Ile-Aib-OH is mainly used in research and development of peptide therapeutics, especially incretin analogs. A prime example is tirzepatide, a 39-amino-acid dual GIP/GLP-1 agonist for diabetes and obesity. Tirzepatide’s sequence contains two Aib residues (positions 2 and 13) that prolong its half-life and albumin binding. Notably, the segment “Ser–Ile–Aib–Leu” appears in tirzepatide’s C-terminal region. By coupling this protected dipeptide into a growing chain, peptide chemists can assemble that Ile–Aib motif efficiently.
Other GLP-1 family peptides also use Aib. For example, semaglutide (a long-acting GLP-1 analog) replaces Ala 8 with Aib in its sequence, enhancing DPP-4 resistance. While semaglutide’s sequence does not have an Ile–Aib pair, it underscores the general role of Aib in peptide drugs. In summary, Fmoc-Ile-Aib-OH finds use in synthesizing GLP-1/GIP analogs and other bioactive peptides where an Ile–Aib sequence is needed.
Solubility and Storage
Fmoc-Ile-Aib-OH should be handled like other peptide intermediates. It is generally not soluble in water. Instead, it dissolves readily in polar aprotic organic solvents used in SPPS (e.g. DMF, DMSO, NMP). Researchers often prepare 0.1–0.5 M solutions in DMF for use in SPPS.
For storage, this compound should be kept cool and dry. It is recommended to store Fmoc-Ile-Aib-OH at around –20 °C and in a desiccated environment. Shielding it from moisture and heat prevents hydrolysis of the Fmoc group or the dipeptide bond. When refrigerated or frozen, it remains stable for extended periods.
Related Peptide Sequences and Use Cases
This building block appears in peptide sequences featuring an Ile–Aib motif or terminal Aib. Notable examples include:
-
Tirzepatide (dual GLP-1/GIP agonist): contains an Ile–Aib sequence (Ser–Ile–Aib–Leu) near positions 11–14.
-
Semaglutide (GLP-1 analog): incorporates Aib at position 8 (Ala→Aib) to resist degradation.
-
Related analogs: Other long-acting peptides and peptide agonists often include Aib residues or Ile–Aib sequences to enhance stability.
For more information and purchase inquiries, please visit PegLinke's official website.

 +8613708084407
+8613708084407