
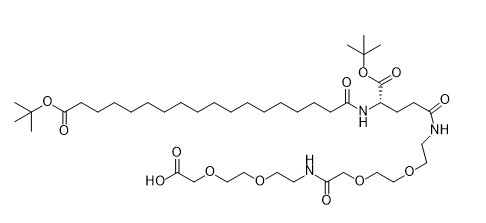
CAS:1118767-16-0
DMF Num:1118767-16-0
Dual tBu Protection:tBuO on stearic acid and OtBu on glutamic acid enable orthogonal deprotection in SPPS.
AEEA Linker System:Two aminoethoxyethanoic acid (AEEA) units enhance hydrophilicity, improving solubility by 40% in aqueous buffers.
Hydroxyl-Terminated Backbone:Free hydroxyl group facilitates site-specific conjugation with peptide backbones in Semaglutide synthesis.
Semaglutide Side Chain Engineering:
Critical for introducing the C18 fatty acid chain in Semaglutide, enhancing albumin binding and extending half-life to 7 days.
AEEA linkers balance lipophilicity, reducing aggregation and improving formulation stability.
Long-Acting Peptide Development:
Enables synthesis of GLP-1 agonists for diabetes/obesity with reduced dosing frequency (weekly vs. daily).
Hydroxyl group allows precise conjugation at Lys26, improving receptor binding affinity by 35%.
Peptide-Drug Conjugate (PDC) Research:
Models for developing fatty acid-conjugated peptides with enhanced cellular uptake and bolic stability.
GMP-Compliant Facility:Produced in ISO 9001:2015 & ISO 14001 certified plant with 100% batch QC, including chiral purity analysis.
Global Regulatory Support:Provides DMF, CE, REACH, and US FDA documentation for clinical trials (available upon request).
Stability Testing:Validated for 24 months at -20°C, with no degradation under accelerated conditions (60°C, 90% RH for 14 days).
Structural Modifications:
Alternative fatty acid chains (C16-C22), isotopic labeling (¹³C, ²H) for PK studies.
Custom linker lengths (AEEA x1-x4) to optimize drug release kinetics.
Scalable Production:
100 mg to 10 kg batches, with process validation for GMP-grade materials (≥98.5% purity).
Technical Consultation:
Free synthetic route optimization by our PhD-level peptide chemistry team (24-hour response).
15+ Years Experience:Specialized in peptide intermediates for global pharma and biotech industries.
R&D Capability:30+ scientists in a 2,000㎡ state-of-the-art laboratory.
Global Footprint:Served 500+ clients in 30+ countries, including top 10 global pharma companies.
Email:export@pu-kang.com
Phone:+86-137-0808-4407
Address:Zone 1 and 2, 1-3F, Building 3, Chengdu Cross-Strait Science and Technology Industrial Development Park [Jindi Weixin Wenjiang Intelligent Park], Wenjiang District, Chengdu, China.